junho 2024
Notícias da América do Norte
The Senate and House of Representatives of the United States of America introduced H.R. 8074, ‘the Forever Chemical Regulation and Accountability Act of 2024’, to phase out all non-essential uses of PFAS in ten years.
On 18 April 2024, the US Federal Government introduced H.R. 8074, ‘the Forever Chemical Regulation and Accountability Act of 2024’ (the FCRAA) to phase out production of nonessential uses of Per- and polyfluoroalkyl substances (PFAS) and to prohibit releases of all PFAS in ten years.
Studies have shown that exposure to certain levels of PFAS may lead to reproductive effects in pregnant women, developmental effects or delays in children, increased risks of some cancers, reduced ability of the body’s immune system to fight infections, and other adverse health effects.
The main contents of the bill include:
Clarification of the key definitions in this Act: essential/nonessential use, National Academies, manufacturer, user, PFAS, etc. PFAS are defined in section 7331(2)(B) of the PFAS Act of 2019 (15 U.S.C.8931(2)(B)): - Perfluoroalkyl substance means a chemical of which all carbon atoms are fully fluorinated. - Polyfluoroalkyl substance means a chemical containing at least one fully fluorinated carbon atom and at least one carbon atom that is not a fully fluorinated carbon atom.
Authorization of the National Academies to: - Review and evaluate the available scientific evidence regarding categories of essential uses of PFAS. - Provide guidance on designating PFAS as essential or nonessential.
Entrance of the Environmental Protection Agency (EPA), in consultation with other Federal departments and agencies, into a 10-year agreement to phase out of nonessential uses of PFAS. The EPA and National Academies may extend the agreement in 5-year increments.
Setting a manufacturing and use phaseout program: - Requires annual PFAS manufacturer and user monitoring and reporting. Amends the Toxic Substances Control Act (TSCA) Section 8 (a)(7) to require manufacturers and users of PFAS to report any essential PFAS usages annually starting no later than 3 years after enactment. - Requiring manufacturer and user to submit a schedule for the full phaseout of nonessential uses of PFAS within the 10-year period not later than 3 years after the date of enactment of this Act. - Identifying an accelerated phase-out in certain products if these goods contain PFAS (see Table 1 below).
Table 1: Accelerated phase-out after the date of enactment of this Act in certain products set out under H.R. 8074.
Regulation: H.R. 8074
| Product/Scope | Requirement | Effective Date |
| Carpets and rugs Fabric treatments Food packaging and containers Juvenile products Oil and gas products | Prohibited if contain PFAS, but exempts items for resale | Phase out within 1 year |
| Accessories and handbags Cosmetics Indoor and outdoor apparel (except for severe wet conditions) Indoor textile furnishings Indoor upholstered furniture | Prohibited if contain PFAS, but exempts items for resale | Phase out within 2 years |
| Outdoor textile furnishings Outdoor upholstered furniture | Prohibited if contain PFAS, but exempts items for resale | Phase out within 4 years |
| Outdoor apparel for severe wet conditions | Prohibited if contain PFAS, but exempts items for resale | Phase out within 5 years |
| All non-essential uses | Prohibited if contain PFAS but exempts for sec.101(h) part | Phase out within 10 years |
The State of Maine has amended its law on products containing intentionally added PFAS. These revisions outlined by S.P. 610 – L.D. 1537 will be implemented in phases, starting in January 2026.
On 16 April 2024, the governor of Maine signed S.P. 610 - LD 1537 into law (Chapter 630) to revise the laws regulating Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) for products containing intentionally added PFAS. As written,
The definition for a ‘carpet or rug’ has been updated to indicate it is a consumer product made from natural or synthetic fabric intended to be used as a floor covering inside commercial or residential buildings. The same product exclusions apply.
Several new terms and product definitions have been added.
The manufacturer is required to advise the Department of Environmental Protection (DEP), via written notification with specific information, if the product contains intentionally added PFAS and in which the DEP has determined that the use of PFAS in the product is currently unavoidable. It should be noted that there is an exception if the manufacturer employs no more than 100 people or has a waiver. This is a change in approach as before the amendment, beginning in 2025, the law required reporting for any product containing intentionally added PFAS.
Additionally, products would not be liable to meet the upcoming 2032 general product prohibition if the DEP determines by rule that the use of PFAS in the product is currently unavoidable.
Highlights of product prohibitions set out under LD 1537 are summarized in the below table.
| Part | Product Scope | Prohibition of PFAS | Effective date | |
|---|---|---|---|---|
| A | Carpet and rug | a) Product with intentionally added PFAS | / | 1-Jan-23 |
| B | Fabric treatment | a) Product with intentionally added PFAS | b) Product that is in a fluorinated container or in a container that contains intentionally added PFAS. | 1-Jan-23 |
| B-1 | •Cleaning products •Cookware •Cosmetics •Dental floss •Juvenile products •Menstruation products •Textile articles, except outdoor apparel for severe wet conditions or those that are part of a watercraft, aircraft or motor vehicle, including off-highway vehicles •Ski wax •Upholstered furniture | a) Product with intentionally added PFAS | b) Product that is in a fluorinated container or in a container that contains intentionally added PFAS. | 1-Jan-26 |
| B-2 | Artificial turfs | a) Product with intentionally added PFAS | b) Product that is in a fluorinated container or in a container that contains intentionally added PFAS. | 1-Jan-29 |
| Outdoor apparel for severe wet conditions | a) Product with intentionally added PFAS | c) Exemption for the apparel if accompanied by the statement "Made with PFAS Chemicals" | ||
| C | Products identified by rule by category or use, especially for those most likely to cause contamination of the State's land or water resources | a) Product with intentionally added PFAS | b) Product that is in a fluorinated container or in a container that contains intentionally added PFAS. | As planned |
| D | Product scope other than part A, B, B-1, B-2, C, and the product that the use of PFAS is a currently unavoidable use. | a) Product with intentionally added PFAS | b) Product that is in a fluorinated container or in a container that contains intentionally added PFAS. | 1-Jan-32 |
| E | •Cooling, heating, ventilation, air conditioning and refrigeration equipment •Refrigerants, foams and aerosol propellants | a) Product with intentionally added PFAS | b) Product that is in a fluorinated container or in a container that contains intentionally added PFAS. | 1-Jan-40 |
The State of Pennsylvania issued Senate Bill No. 756 which amends the Act of 25 July 1961 (P.L.0857, No.0372), known as the Stuffed Toy Manufacturing Act.
The Stuffed Toy Manufacturing Act, P.L. 0857, No. 0372, was passed in the State of Pennsylvania in 1961 and did not contemplate the use of recycled material in stuffed toys.
Senate Bill No. 756 was put forth by the General Assembly of Pennsylvania, to address this information, and thereby amend this Act.
Manufacturing processes and consumer preferences have undergone changes since 1961. The use of recycled materials in stuffed toys has become more prevalent and widely accepted. Many manufacturers and retailers have set sustainability goals for their products in which using recycled materials is imperative. Additionally, consumers have become more environmentally conscious and prefer to purchase items made from recycled materials to lessen their environmental footprint.
Using recycled materials in products is safe for consumers. Recycled materials that are currently used in stuffed toys go through the same extensive testing and safety review as toys manufactured using new materials and can meet all current industry standards.
Notable section highlights referenced in Senate Bill No. 756 include:
-Section 5. All material used in stuffed toys shall be new or recycled material and free from dangerous or harmful chemicals or other substances and shall be free from oil, dirt, refuse and similar substances.
-Section 3. The act is amended by adding a section to read:
Section 9.1.
(a) Each stuffed toy manufactured for sale, delivered, consigned or possessed for sale, sold or offered for sale, gift or use in this Commonwealth shall have securely affixed to it a tag or label. The form, design, color or size of the label is left to the discretion of the manufacturer or importer. The information required on the label shall be clearly legible and in sufficient size type so that it may be readily discerned.
(b) The label of a stuffed toy shall bear the following information:
(1) A statement that the type of material used in the manufacture of the stuffed toy is new, recycled or a mix of new and recycled materials.
(2) The registration number of the manufacturer or importer assigned by the Commonwealth preceded by the abbreviations "REG. NO. PA."
(c) No person other than the one granted a given registration or the person's designated agent shall use the registration number.
Reference: https://www.senate.mo.gov/24info/pdf-bill/intro/SB756.pdf
On 29 May 2024, the new settlement for BPA in socks designed for females and made of spandex or 51% or more polyester was finalized under the County of San Francisco Case No. CGC-22-598022 and CGC-22-602383.
To ensure compliance with Proposition 65, on and after the compliance date, companies doing business in California shall not manufacture, distribute or sell any covered product in California that contains Bisphenol A (BPA) exceeding 10 ppm, except sell-through for existing inventory. The settlement date was 29 May 2024. The compliance date will be 29 May 2025.
The main contents of the new settlement are:
Covered Product: socks designed for females whose composition includes spandex and 51% or more polyester.
BPA requirement: - BPA in covered product shall not exceed 10 ppm as measured by the Test Protocol. - There is no mandatory requirement to test for the presence of other bisphenols. - Other bisphenols mean Bisphenol AF (BPAF), Bisphenol AP (BPAP), Bisphenol B (BPB), Bisphenol E (BPE), Bisphenol F (BPF), Bisphenol P (BPP), Bisphenol S (BPS), Bisphenol Z (BPZ). - Reminder: Bisphenol S (BPS) was also listed in Prop 65 on 29 Dec 2023.
Test method: The specific test protocol is nominated in EXHIBIT A of the settlement, in which BPA is tested with reference to the various EPA methods, with a reporting limit of 0.5 mg/kg or lower.
Regulations Amending Certain Regulations Concerning the Disclosure of Cosmetic Ingredients: SOR/2024-63 were published on 24 April 2024.
The key objectives were to:
Introduce a requirement to disclose certain fragrance allergens on cosmetic labels.
Provide greater flexibility for disclosure of ingredients for cosmetics sold in small packages
Improve oversight of cosmetics
Make administrative updates
On 24 April 2024, Regulations Amending Certain Regulations Concerning the Disclosure of Cosmetic Ingredients: SOR/2024-63 were published in the Canada Gazette, Part II under the FOOD AND DRUGS ACT and CANNABIS ACT.
In summary, the amended regulations include the below:
Disclosure of Fragrance Allergens:
The amendments require the disclosure of certain fragrance allergens on cosmetic labels when present above a specified limit (0.01% for rinse-off products, 0.001% for leave-on products).
This aligns with the European Union's requirements and uses an ambulatory incorporation by reference approach.
The amendments also include a transition period aligned with the European Union’s timelines.
Flexibility for Small Packages:
The amendments provide the option to disclose the list of ingredients on a website for cosmetics sold in small packages, in addition to the current options of a tag, tape or card.
This is intended to address challenges with legibility of labels on small packages.
Improved Oversight:
The amendments update the definition of "manufacturer" and add a definition for "importer" to improve clarity.
Notification requirements are strengthened, including clarifying the timelines for responding to information requests.
The amendments give Health Canada the ability to issue a stop sale if notification requirements are not met.
Ingredient disclosure on notifications must use INCI names, and concentration ranges are expanded.
Health Canada can request safety evidence from importers, not just manufacturers.
Administrative Updates:
Various definitions and references are updated for clarity and consistency.
The schedule of ingredient names is revised.
Implementation and Coming into Force:
The amendments come into force in two stages:
All amendments except fragrance allergen disclosure - 180 days after registration, by 9 October 2024
Fragrance allergen disclosure requirements - 2 years after registration, on 12 April 2026
In the US, when hazards are identified in consumer products, they will be recalled and published in the Consumer Product Safety Commission (CPSC) Recent Recalls on the CPSC website, which is updated daily. The US recalls from 01 May 2024 to 31 May 2024 are summarized below:
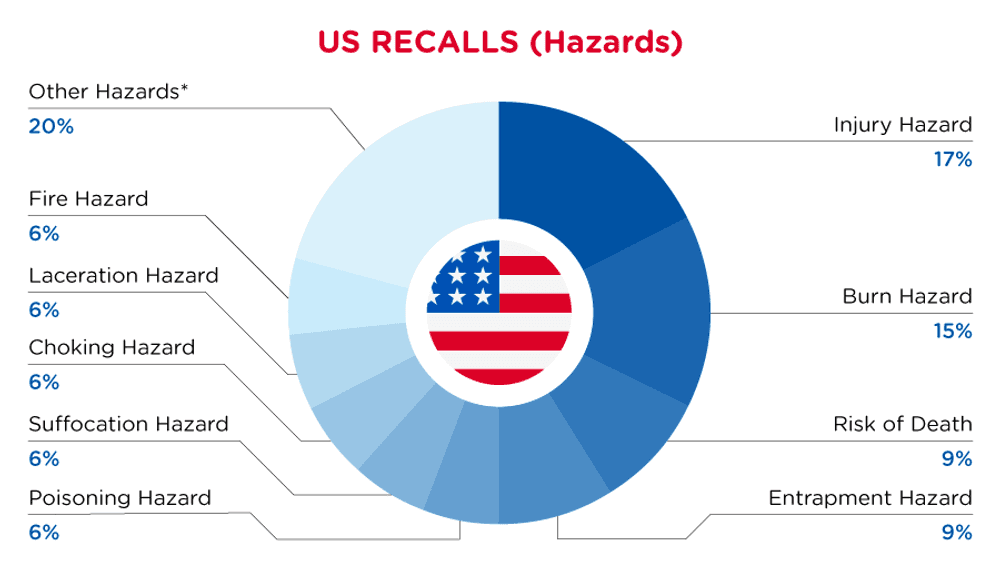
| Hazards | Frequency |
| Injury Hazard | 6 |
| Burn Hazard | 5 |
| Risk of Death | 3 |
| Entrapment Hazard | 3 |
| Poisoning Hazard | 2 |
| Suffocation Hazard | 2 |
| Choking Hazard | 2 |
| Laceration Hazard | 2 |
| Fire Hazard | 2 |
| Other Hazards* | 7 |
*Other Hazards include Microbiological Hazard, Swallowing Risk, Health Risk Hazard, Chemical Hazard, Fall Hazard, Tip-Over Hazard and Impact Hazard with a frequency of less than 2.
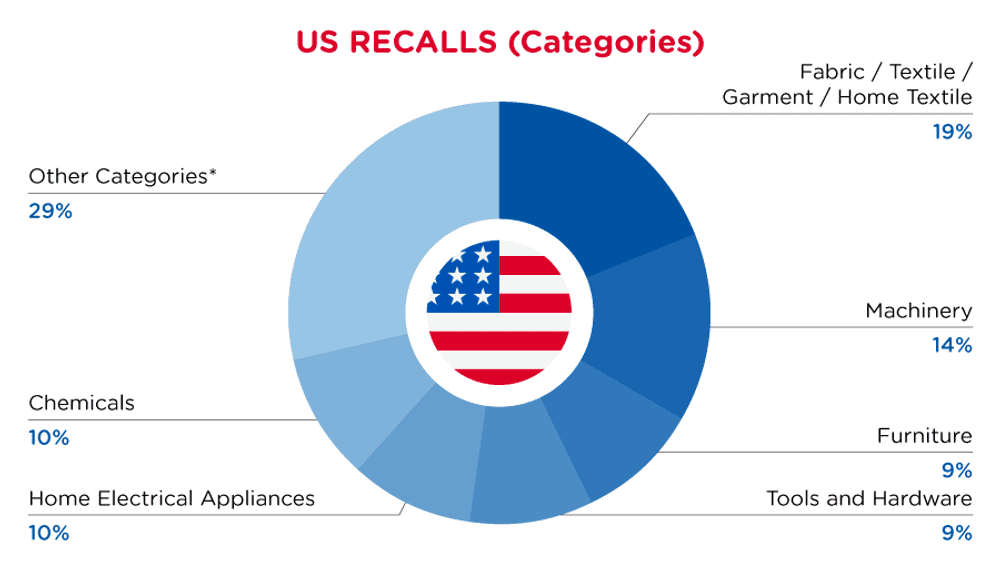
| Product Categories | Frequency |
| Fabric / Textile / Garment / Home Textile | 4 |
| Machinery | 3 |
| Furniture | 2 |
| Tools and Hardware | 2 |
| Home Electrical Appliances | 2 |
| Chemicals | 2 |
| Other Categories* | 6 |
*Other Categories include Toys and Childcare Products, Pet Items, Protective Equipment, Food Contact Material, Household Items and Electrical Appliances with a frequency of less than 2.
For a complete list click here
In Canada, when hazards are identified in consumer products, they will be recalled and published in the Recalls and Safety Alerts Database on the Health Canada website, which is updated daily. The Canada recalls from 01 May 2024 to 31 May 2024 are summarized below:
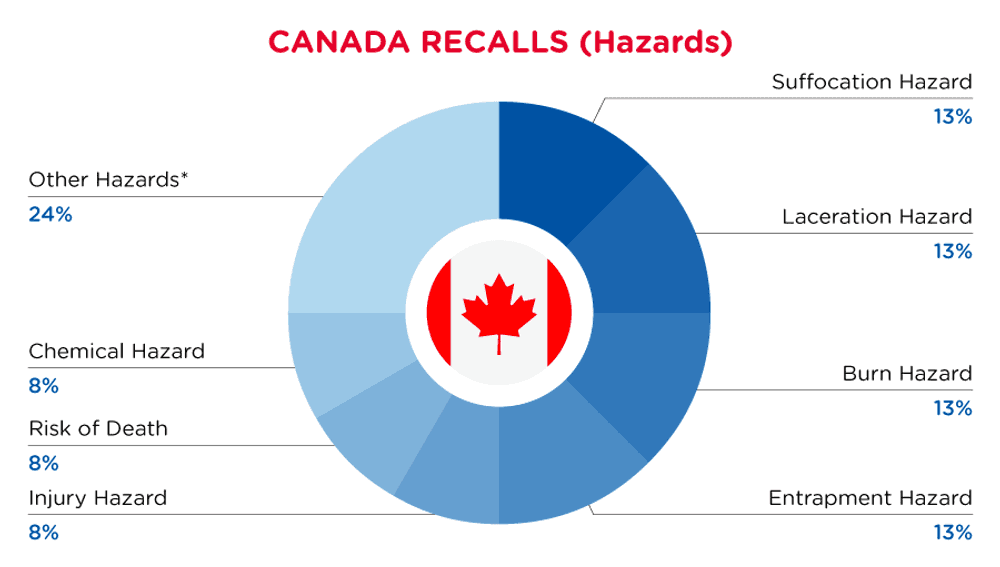
| Hazards | Frequency |
| Suffocation Hazard | 3 |
| Laceration Hazard | 3 |
| Burn Hazard | 3 |
| Entrapment Hazard | 3 |
| Injury Hazard | 2 |
| Risk of Death | 2 |
| Chemical Hazard | 2 |
| Other Hazards* | 6 |
*Other Hazards include Safety Risk Hazard, Ingestion Hazard, Entanglement Hazard, Choking Hazard, Strangulation Hazard and Fire Hazard with a frequency of less than 2.
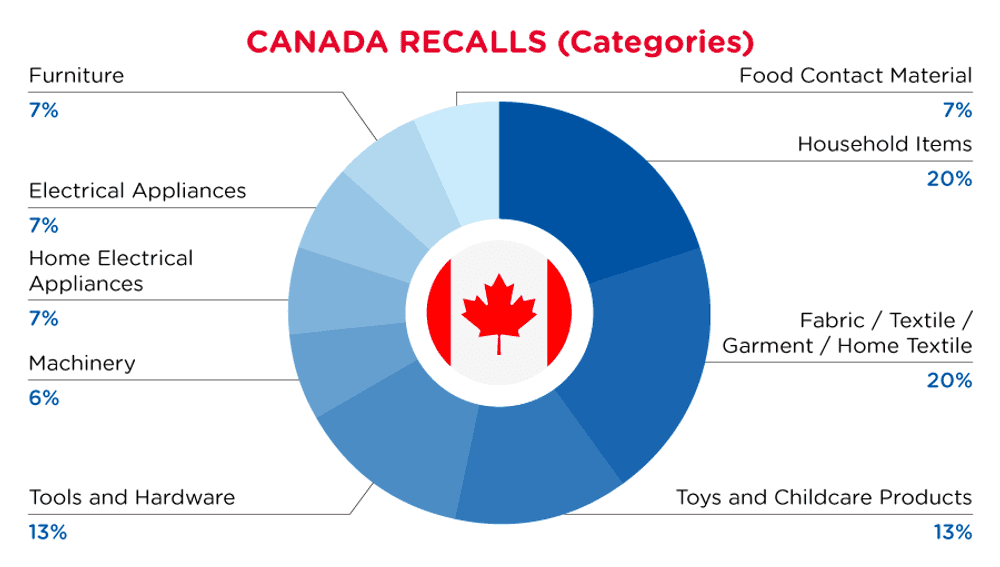
| Product Categories | Frequency |
| Household Items | 3 |
| Fabric / Textile / Garment / Home Textile | 3 |
| Toys and Childcare Products | 2 |
| Tools and Hardware | 2 |
| Machinery | 1 |
| Home Electrical Appliances | 1 |
| Electrical Appliances | 1 |
| Furniture | 1 |
| Food Contact Material | 1 |
For a complete list click here
Notícias da Europa
Commission Regulation (EU) 2024/1328 of 16 May 2024, amends Entry 70 of Annex XVII to Regulation (EC) No 1907/2006, expanding the restriction of substance and product scope with a phased effective date approach. The regulation is effective after 6 Jun 2024.
With the amendment of Regulation (EU) 2024/1328, Entry 70 of Annex XVII to REACH Regulation adds the restriction of the substance Dodecamethylcyclohexasiloxane (D6) (CAS No 540-97-6) and expands the restriction of product scope with phased effective dates.
The main restrictions are: (see Table 1)
Octamethylcyclotetrasiloxane (D4), CAS No 556-67-2
Decamethylcyclopentasiloxane (D5), CAS No 541-02-6
Dodecamethylcyclohexasiloxane (D6), CAS No 540-97-6
Table 1.
| Substances | Product/Scope | Requirement | Effective date |
|---|---|---|---|
| (D4) (D5) | Wash-off cosmetic products as defined in Article 2(1), point (a), of Regulation (EC) No 1223/2009 in a concentration of D4 or D5 ≥ 0,1 % by weight | Prohibited | 31 January 2020 (as in existing law) |
| (D4) (D5) (D6) | all cosmetic products other than the ones mentioned above | Prohibited | 6 June 2027 |
| (D4) (D5) (D6) | as a substance on its own as a constituent or in mixtures with concentration≥0,1 % by weight of the respective substance | Prohibited | 6 June 2026 |
| (D4) (D6) | as a solvent for the dry cleaning of textiles, leather and fur | Prohibited | 6 June 2026 |
| (D5) | for D5 as a solvent in the dry cleaning of textiles, leather and fur with a constituent or in mixtures≥0,1 % by weight | Prohibited | 6 June 2034 |
| (D4) (D5) (D6) | medical devices as defined in Article 1(4) of Regulation (EU) 2017/745 and in Article 1(2) of Regulation (EU) 2017/746 medicinal products, as defined in Article 1, point 2, of Directive 2001/83/EC, and for veterinary medicinal products, as defined in Article 4(1) of Regulation (EU) 2019/6 | Prohibited | 6 June 2031 |
With the amendment of Regulation (EU) 2024/1328, Entry 70 of Annex XVII to REACH Regulation adds the restriction of the substance Dodecamethylcyclohexasiloxane (D6) (CAS No 540-97-6) and expands the restriction of product scope with phased effective dates.
The main restrictions are: (see Table 1)
Octamethylcyclotetrasiloxane (D4), CAS No 556-67-2
Decamethylcyclopentasiloxane (D5), CAS No 541-02-6
Dodecamethylcyclohexasiloxane (D6), CAS No 540-97-6
Table 1.
| Substances | Product/Scope | Requirement | Effective date |
| (D4) (D5) | Wash-off cosmetic products as defined in Article 2(1), point (a), of Regulation (EC) No 1223/2009 in a concentration of D4 or D5 ≥ 0,1 % by weight | Prohibited | 31 January 2020 (as in existing law) |
| (D4) (D5) (D6) | all cosmetic products other than the ones mentioned above | Prohibited | 6 June 2027 |
| (D4) (D5) (D6) | as a substance on its own as a constituent or in mixtures with concentration≥0,1 % by weight of the respective substance | Prohibited | 6 June 2026 |
| (D4) (D6) | as a solvent for the dry cleaning of textiles, leather and fur | Prohibited | 6 June 2026 |
| (D5) | for D5 as a solvent in the dry cleaning of textiles, leather and fur with a constituent or in mixtures≥0,1 % by weight | Prohibited | 6 June 2034 |
| (D4) (D5) (D6) | medical devices as defined in Article 1(4) of Regulation (EU) 2017/745 and in Article 1(2) of Regulation (EU) 2017/746 medicinal products, as defined in Article 1, point 2, of Directive 2001/83/EC, and for veterinary medicinal products, as defined in Article 4(1) of Regulation (EU) 2019/6 | Prohibited | 6 June 2031 |
The main derogations or allowances are:
Placing on the market of D4, D5 and D6 for the industrial uses of silicone polymer/ non-metal surface treatment.
D4, D5 or D6 in a concentration ≤ 1 % by weight of the respective substance in the mixture, for use in adhesion, sealing, gluing and casting.
D4 in a concentration ≤ 0,5 % by weight, or D5 or D6 in a concentration≤ 0,3 % by weight of either substance in the mixture for use as protective coatings.
Limited usage for professional and laboratory use, some medical devices, silicone insoles for horses, adhesion promoters, 3D-printing, pad printing, etc.
D5 as a solvent in strictly controlled closed dry-cleaning systems for textile, leather and fur, where the cleaning solvent is recycled or incinerated.
In March 2024, ECHA announced a consultation phase for potential inclusion of two chemicals as Substances of Very High Concern (SVHC). After the consultation period, the SVHC Candidate List was updated to include Bis(α,α-dimethylbenzyl) peroxide, bringing the total number of entries to 241.
After the consultation period, ECHA decided to update the SVHC Candidate List by including only bis(α,α-dimethylbenzyl) peroxide [CAS number: 115-86-6], bringing the total number of entries to 241 on 27 June 2024.
Details of the new addition to the SVHC Candidate List, Bis(α,α-dimethylbenzyl) peroxide, are summarized below.
| Substance name | EC number | CAS number | Reason for inclusion | Example of usage |
|---|---|---|---|---|
| Bis(α,α-dimethylbenzyl) peroxide | 201-279-3 | 80-43-3 | Toxic for reproduction (Article 57c) | Flame retardant |
ECHA announced that, for Triphenyl phosphate, inclusion in the SVHC candidate list is suspended. This is based on substantial new information which recently became available.
The suspension for Triphenyl phosphate in this round of updates aims to ensure the new information provided for this chemical will be properly evaluated and considered in the SVHC identification process.
In Europe, when hazards are identified in non-food consumer products, the products will be recalled and published in the Safety Gate system, which is updated weekly. The European recalls from 01 May 2024 to 31 May 2024 are summarized below:
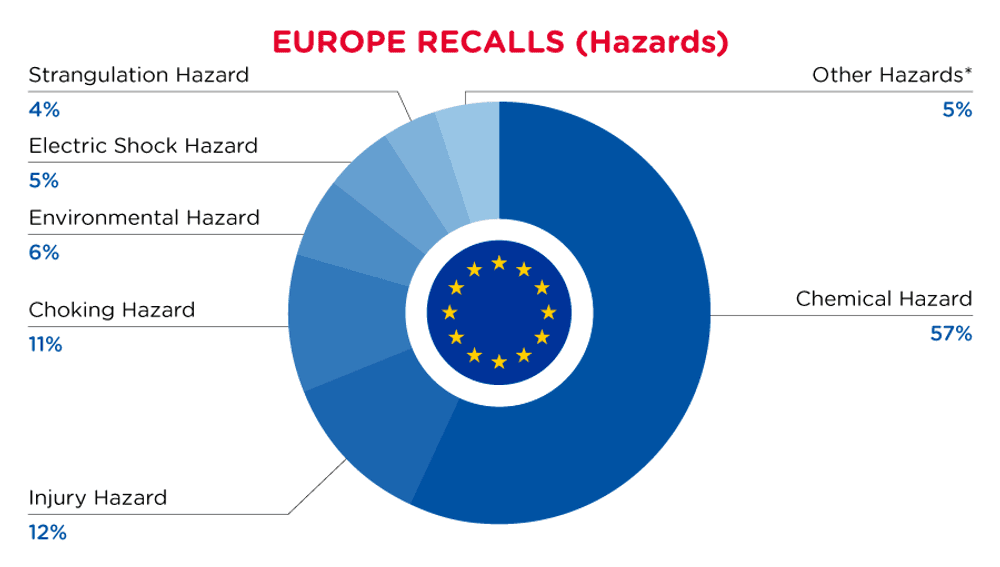
| Hazards | Frequency |
| Chemical Hazard | 216 |
| Injury Hazard | 45 |
| Choking Hazard | 40 |
| Environmental Hazard | 23 |
| Electric Shock Hazard | 20 |
| Strangulation Hazard | 16 |
| Other Hazards* | 18 |
*Other Hazards include Fire Hazard, Burn Hazard, Suffocation Hazard, Cut Hazard, Health Risk Hazard, Entrapment Hazard and Explosion Hazard with a frequency of less than 16.
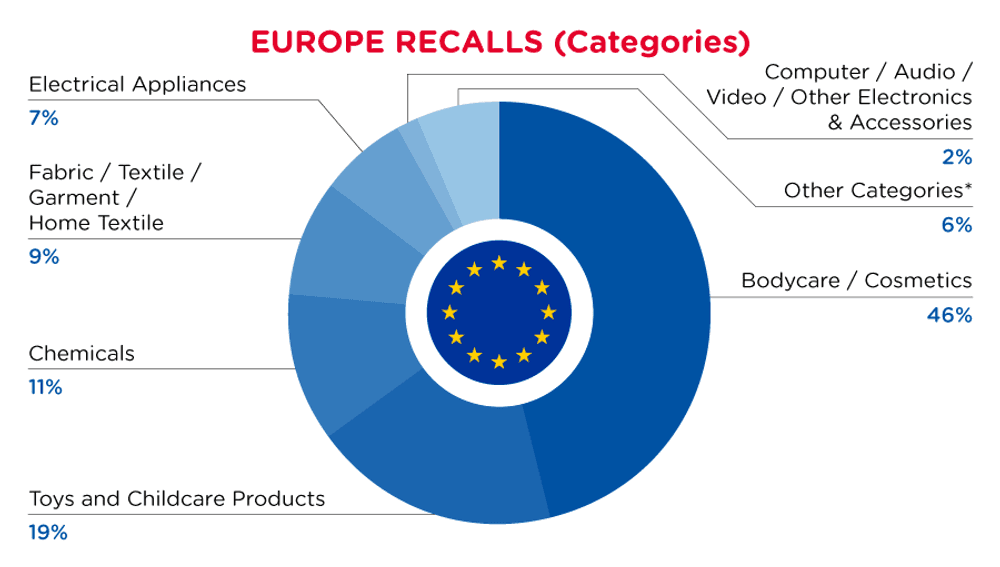
| Product Categories | Frequency |
| Bodycare / Cosmetics | 160 |
| Toys and Childcare Products | 65 |
| Chemicals | 39 |
| Fabric / Textile / Garment / Home Textile | 31 |
| Electrical Appliances | 23 |
| Computer / Audio / Video / Other Electronics & Accessories | 5 |
| Other Categories* | 22 |
*Other Product Categories include Stationery, Furniture, Jewelry, Car Accessories, Protective Equipment, Home Electrical Appliances, Sporting Goods / Equipment, Footwear, Machinery, Household Items and Tools and Hardware with a frequency of less than 5.
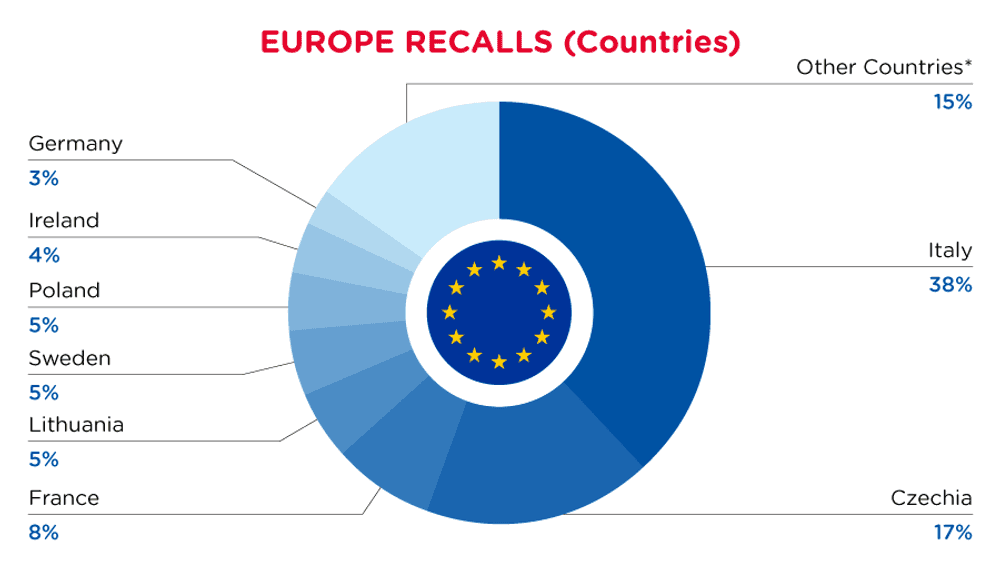
| Notifying Country | Frequency |
| Italy | 132 |
| Czechia | 60 |
| France | 27 |
| Lithuania | 18 |
| Sweden | 17 |
| Poland | 16 |
| Ireland | 13 |
| Germany | 10 |
| Other Countries* | 52 |
*Other Countries include Belgium, Finland, Romania, Austria, Bulgaria, Hungary, The Netherlands, Cyprus, Denmark, United Kingdom in respect of Northern Ireland, Luxembourg, Croatia, Latvia, Malta and Slovakia with a frequency of less than 10.
For a complete list click here
Notícias da Austrália/Nova Zelândia
In Australia, when hazards are identified in consumer products, they will be recalled and published in the Recalls and Safety Alerts Database on the Australian Competition & Consumer Commission website, which is updated daily. The Australia recalls from 01 May 2024 to 31 May 2024are summarized below:
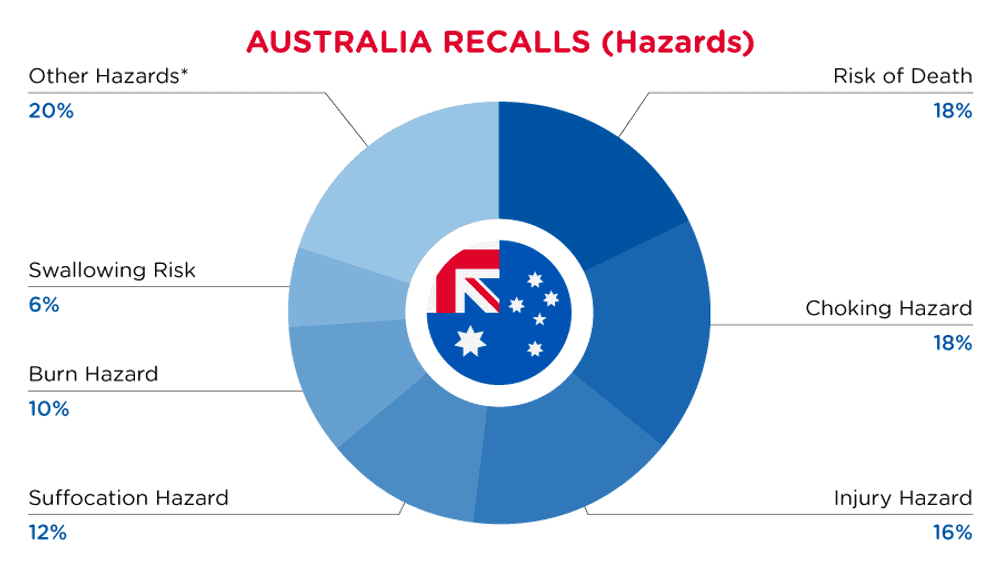
| Hazards | Frequency |
| Risk of Death | 9 |
| Choking Hazard | 9 |
| Injury Hazard | 8 |
| Suffocation Hazard | 6 |
| Burn Hazard | 5 |
| Swallowing Risk | 3 |
| Other Hazards* | 10 |
*Other Hazards include Fire Hazard, Damage to Sight, Electric Shock Hazard, Cut Hazard, Entanglement Hazard, Health Risk Hazard, Skin Irritation Risk, Laceration Hazard and Eye Irritation Risk with a frequency of less than 3.
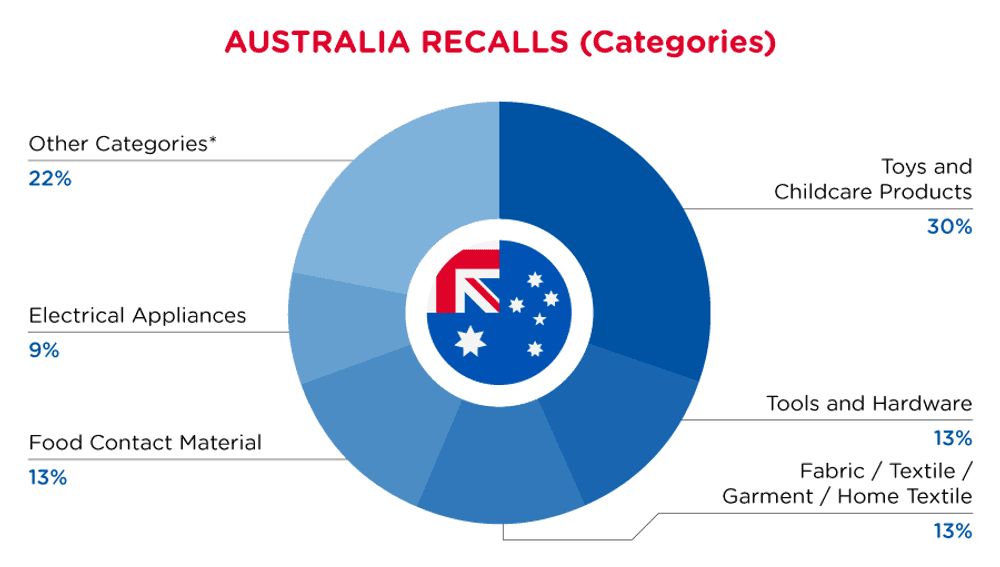
| Product Categories | Frequency |
| Toys and Childcare Products | 7 |
| Tools and Hardware | 3 |
| Fabric / Textile / Garment / Home Textile | 3 |
| Food Contact Material | 3 |
| Electrical Appliances | 2 |
| Other Categories* | 5 |
*Other Categories include Sporting Goods / Equipment, Jewelry, Bodycare / Cosmetics, Computer / Audio / Video / Other Electronics & Accessories and Chemicals with a frequency of less than 2.
For a complete list click here
Assine nossas Atualizações Regulatórias
Cancelar a assinatura a qualquer momento. Leia nossa política de privacidade.